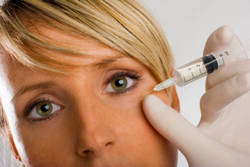
 The FDA has approved BOTOX® neurotoxin to decrease the severity of abnormal head position and neck pain associated with cervical dystonia (CD; also known as spasmodic torticollis).
The FDA has approved BOTOX® neurotoxin to decrease the severity of abnormal head position and neck pain associated with cervical dystonia (CD; also known as spasmodic torticollis).
BOTOX® is also FDA approved to treat blepharospasm (eyelid spasms), and strabismus (crossed eyes), primary axillary hyperhidrosis (excessive sweating), chronic migraine headaches (>14 headache days a month) and upper limb spasticity seen patients with stroke, brain injury, multiple sclerosis, cerebral palsy or spinal cord injury. In fact, BOTOX® was originally used to target medical problems before its popularity in cosmetics.
Once you receive BOTOX® neurotoxin treatment, you’ll start to feel the benefits soon, usually within a few days to a couple of weeks after receiving BOTOX®. This relief may last for up to 3 months. BOTOX® injections are not performed more frequently than 3 months apart inorder to avoid the body’s natural tendency to develop antibodies which can make you resistant to future treatments.


